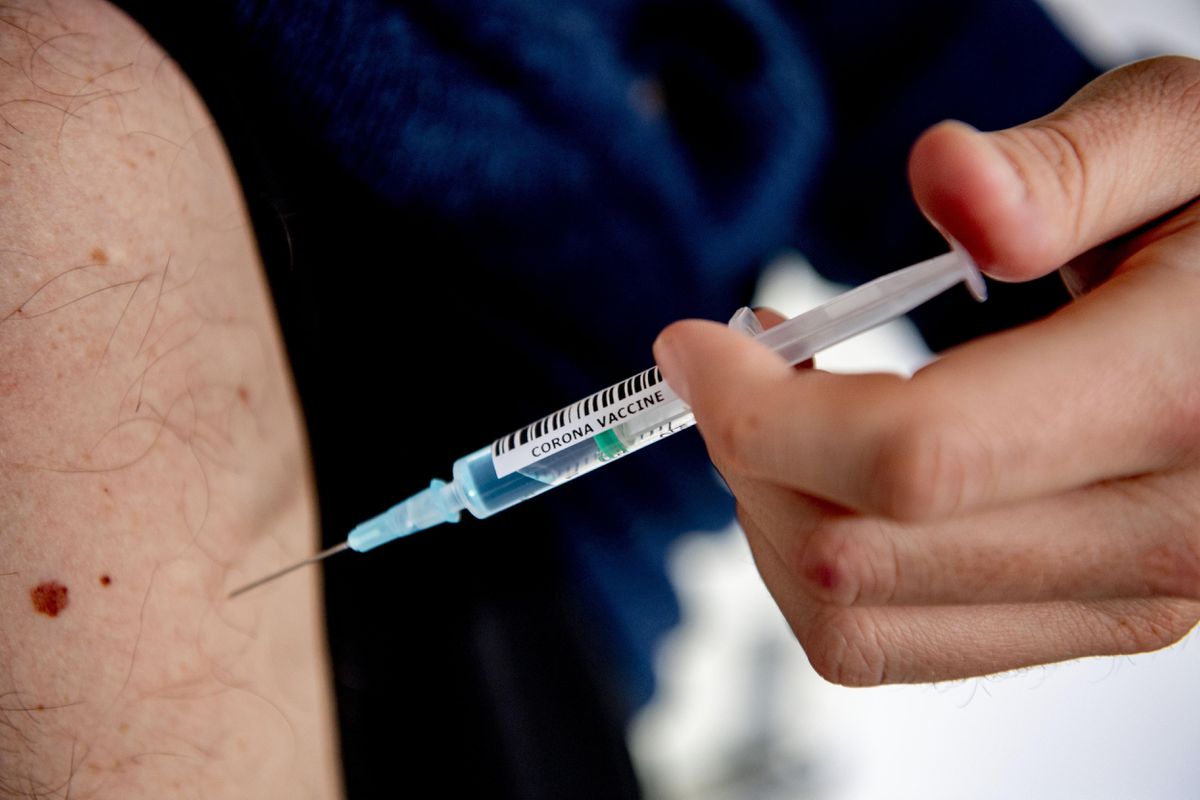
The EU pharmaceutical regulator will carry out its formal evaluation of the Pfizer/BioNTech and Modern Covid-19 vaccine on 29 December and 12 January after the two applicants were implemented for EU approval, the timing of which is likely to boost the availability of vaccine opposed to coronavirus in Europe until 2021.
The European Medicines Agency (EMA) will convene an assembly on 29 December to compare knowledge of the Pfizer vaccine trial, while the resolution on the Modern vaccine is expected to take place on 12 January, the signing was announced in separate press releases.
The evaluation dates announced through the EMA appear to be a delay, as the vaccines were scheduled to be evaluated first on December 22, reported by the Financial Times, which brought internal documents.
Ursula von der Leyen, president of the European Commission, had even said that if all went well, “the first European citizens can be vaccinated before the end of December. “
Approval of any vaccine by Member States will take place 3 to 4 days after the vaccine is approved through the EMA, meaning vaccines are unlikely to be available on the block until early January.
According to the FT, the two vaccine brands completed their submission to the EMA on Monday, which has been reviewing knowledge of the trial on an ongoing basis since October.
The less expensive and easiest-to-manufacture AstraZeneca/Oxford will not be tested until January, the FT report adds.
In any case, the EMA will take into account a conditional marketing authorization (CMA) for vaccines, to an emergency use authorization from the US FDA. Usa, which is an early authorization based on initial knowledge that demonstrates that the benefits of the vaccine outweigh any possible Rique. The knowledge presented through Moderna and Pfizer shows that vaccines are 94% and 95% effective in disease prevention.
Two billion. This is the total number of doses of a Covid-19 vaccine that the 27 Member States of the European Union have controlled to download with deliveries probably from the end of 2020. The agreement includes dosages from vaccine manufacturers, adding Moderna, Pfizer/BioNTech, AstraZeneca, Sanofi-GSK, Janssen Pharmaceutica NV and CureVac.
Pfizer partner BioNTech said it was able to send stock of vaccines where they are needed when the company approved the vaccine. “Depending on how the government can start delivering in a matter of hours,” Sierk Poetting, BioNTech’s executive leader, told The Associated Press.
Officials from the bloc’s member states insisted that the EMA align its approval with that of the United States and Britain. In August, the British government announced that it would grant emergency approval to a convincing candidate vaccine even before the EMA did. Although the UK is still subject to the EMA’s regulatory procedure until 31 December, when the transition to Brexit will be completed, Pfizer and Modern sent their vaccines to the US FDA. U. S. for emergency use authorization. Vice President Mike Pence told governors that the distribution of the vaccine is expected to begin in mid-December.
Regulator of the eu to the first vaccine opposed to the virus on December 29 (Associated Press)
Covid vaccines in the EU are expected to begin until next year (Financial Times)
Full policy and updates on Coronavirus
I am a reporter for Breaking News in Forbes, with a policy of generating coverage and vital commercial news. Graduated from Columbia University with a master’s degree in business and
I am a reporter for Breaking News in Forbes, with a policy of generating coverage and vital commercial news. Graduated from Columbia University with a master’s degree in economic and business journalism in 2019. I worked as a journalist in New Delhi, India, from 2014 to 2018. Do you have any advice?DVs are open on Twitter @SiladityaRay or email me at siladitya@protonmail. com.